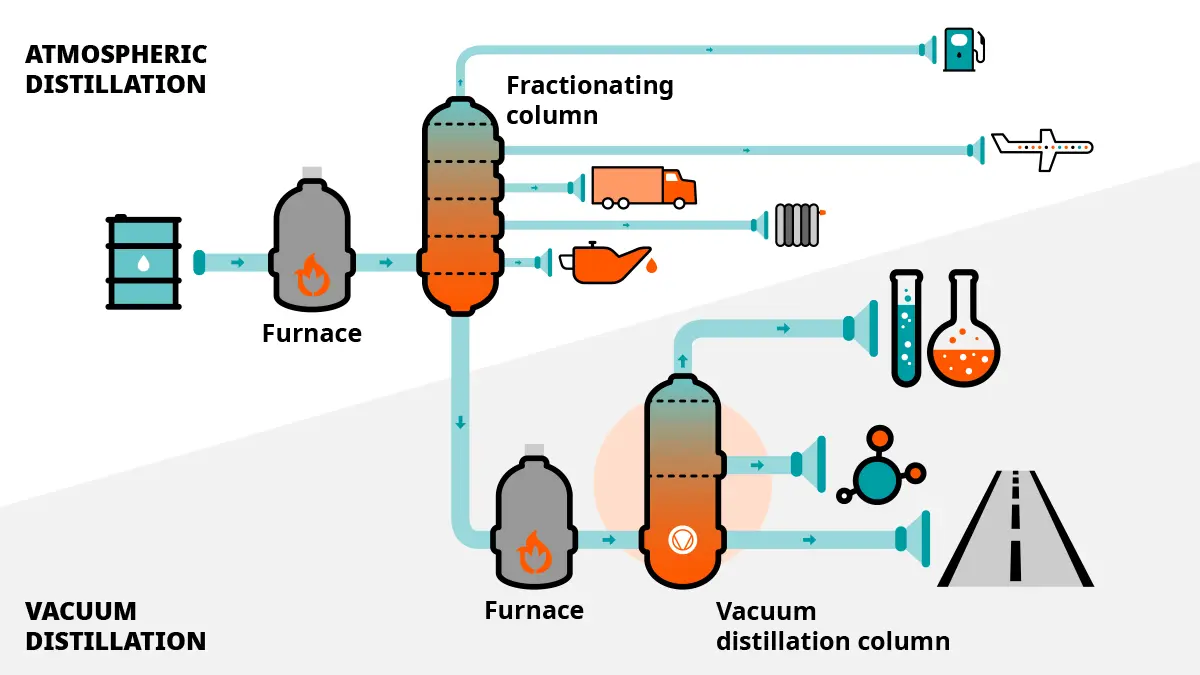
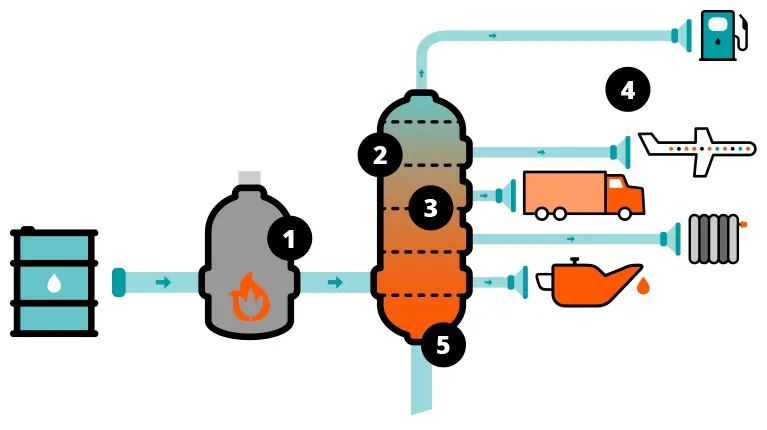
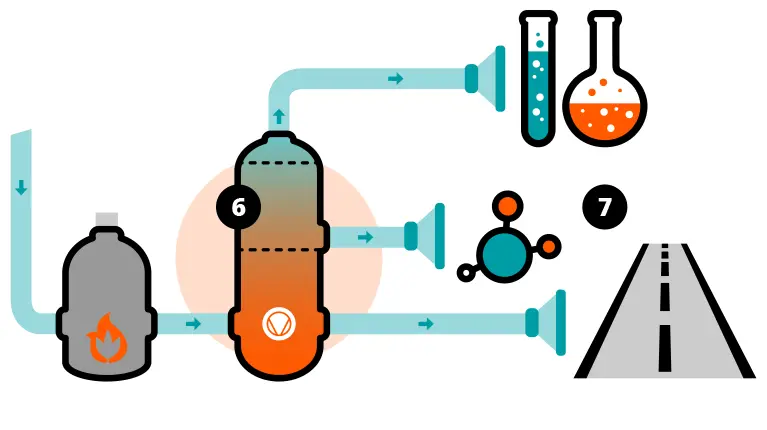
The role of vacuum in crude oil distillation
When it is extracted from the ground, crude oil is a mixture of many different liquid hydrocarbons.However, to ensure that there are no unwanted byproducts and no degrading, the oil must be kept below a certain temperature.
Under vacuum, the boiling point of liquids can be lowered. Vacuum distillation therefore ensures that the different types of oil contained in the crude oil mixture can be separated at a lower temperature.